Blood borne Pathogens
Blood borne pathogens are microorganisms such as viruses or bacteria that are carried in blood and can cause disease in people.Blood borne Pathogen control in the workplace is an essential program to keep employees safe if they are required to provide first aid care in the workplace.
The viruses that cause Hepatitis B Virus (HBV) and Human Immune-deficiency Virus (HIV) are two examples of blood borne pathogens. For a blood borne pathogen to be spread, the bodily fluids of an infected person must enter into the bloodstream of another person. The most common cause of transmission in the workplace is when an infected person’s blood enters another person’s bloodstream through an open wound.
Occupational Exposure:
The Occupational Safety and Health Administration (OSHA) standard 29 CFR 1910.1030(c)(1)(i) states that “Each employer having an employee(s) with occupational exposure as defined by paragraph (b) of this section shall establish a written Exposure Control Plan designed to eliminate or minimize employee exposure.”
Occupational exposure, as defined by paragraph (b), is “reasonably anticipated skin, eye, mucous membrane, or parenteral contact with blood or other potentially infectious materials that may result from the performance of an employee’s duties.”
There are good reasons to provide workers with a safety training program about bloodborne pathogens. First, anybody can be exposed during an accident or even from close contact with someone who has an open sore. Second, OSHA has cited contractors for failing to provide education in this area, specifically when employees are required to be certified in first aid/CPR. And third, business-owned facilities, such as shops and offices, are covered under the general industry regulations and therefore subject to OSHA’s bloodborne pathogen standard.
Definitions:
Following are some terms that appear in this section that are critical to the understanding of bloodborne pathogens.
Bloodborne pathogens: Microorganisms that are present in human blood and can cause disease in humans. These pathogens include, but are not limited to, HBV and HIV.
Engineering controls: Controls that isolate or remove bloodborne pathogens from the workplace. exposure incident: A specific eye, mouth, other mucous membrane, nonintact skin, or parenteral contact with blood or other potentially infectious materials that result from the performance of an employee’s duties.
Hepatitis B Virus (HBV): The most common form of hepatitis; a liver disease that initially causes inflammation of the liver and frequently leads to more serious conditions, including cirrhosis and liver cancer. HBV is usually transmitted through mucous membranes or breaks in the skin. After exposure, it can take two to six months for HBV to develop. The initial symptoms of HBV infection are like those of a mild case of the flu: fatigue, stomach pain, loss of appetite and nausea. As the disease progresses, jaundice (yellowing of the skin) and darkened urine will occur. Although there is no cure, vaccination directly after contact (well before symptoms appear) can prevent infection.
Human Immunodeficiency Virus(HIV): A bloodborne pathogen that attacks the immune system. Symptoms of HIV can include weakness, fever, sore throat, nausea, headaches, diarrhea and some forms of cancer. Many people can go years before showing any symptoms. HIV eventually may lead to Acquired Immune Deficiency Syndrome (AIDS) and the breakdown of the immune system. Currently, there is no vaccination against HIV and no proven cure. However, there have been some major breakthroughs in recent years in controlling HIV and significantly delaying the onset of AIDS.
Other pathogens covered by the standard:
Other bloodborne pathogens are covered by the standard. Some of these are infectious diseases that are characterized by a phase in which the virus or bacteria causing the disease may circulate in the blood for a prolonged period. They are therefore capable of being transmitted through blood or other potentially infectious materials. With the exception ofsyphilis and malaria, they are rare in the United States. The following is a list of some other bloodborne pathogens that are also covered by the standard:
1. Syphilis
2. Malaria
3. Babesiosis
4. Brucellosis
5. Leptospirosis
6. Arboviral infections (especially Colorado tick fever)
7. Relapsing fever
8. Creutzfeldt-Jakob disease
9. Human T-lymphotropic virus type I
10. Viral hemorrhagic fever
Exposure Control Plan:
 Who must have an exposure control plan?
Who must have an exposure control plan?
Any employer with employees covered by the standard must have a written exposure control plan. This includes allemployers with employees who may have occupational exposure to blood. If should be noted that plans are required of employers with two categories of employees other than those most readily identifiable:
(1) part-time, temporary and perdiem employees in the health care industry and
(2) employees trained in first aid/CPR and designated to respond to emergencies in any place of employment.
What must the exposure control plan include?
The exposure control plan must include a determination of which employees are covered using
(1) a list of all job classificationin which all employees have occupational exposure to blood or other potentially infectious materials and
(2) a list of job classifications in which only some of the employees have occupational exposure. The job classifications in which only some of the employees have exposure must then be analyzed. Those tasks and procedures in which occupational exposure occurs must be identified, and the employees performing those tasks, and therefore covered by the plan, identified. However, it is not necessary to list individual employee names.
The exposure determination must be made without regard to the use of personal protective equipment (as though it were not being used). An exposure control plan is required of all employers with employees covered by the standard.
In addition to the determination of covered employees outlined above, it must include at least the following elements:
1. The schedule and methods of implementation for all elements of the standard that pertain to this employer.
2. The procedure for the evaluation of circumstances surrounding exposure incidents, as required by the standard.
The standard requires the employer to annually review and update the exposure control plan and more frequently when necessary to reflect new or modified tasks that affect occupational exposure as well as to reflect new or revised employee positions with occupational exposure. An important part of this review is the consideration and evaluation of engineering controls, such as commercially available safer needle devices and needleless systems, which eliminate or reduce employee exposure to percutaneous injury with contaminated devices.
To accomplish this, the employer must solicit input from nonmanagerial employees in the evaluation, selection, and use of new commercially available devices. The nonmanagement employees chosen must be from among those who are responsible for direct patient care.
A copy of the standard with notes detailing the schedule and method of implementation of the standard in that particular workplace may be adequate for small facilities. Larger facilities may wish to incorporate the exposure control plan as one portion of the infection control plan or may otherwise develop a facility-wide program. Model exposure control plans, which can be adapted to individual company needs, can be obtained either through the Education, Training.
Methods of Compliance:
Universal precautions:
Universal precautions, as outlined and defined by the Centers for Disease Control and Prevention (CDC), are to be used to prevent contact with blood or other potentially infectious materials. The term universal precautions refers to a method of bloodborne disease control which requires that all human blood and other potentially infectious materials be treated as if known to be infectious with HIV, HBV or other bloodborne pathogens regardless of the perceived low risk of a patient or patient population. Another method of infection control is called body substance isolation (BSI) or standard precautions.
This method defines all body fluids and substances as infectious. BSI incorporates not only the fluids and materials covered by this standard but expands coverage to include all body fluids and substances. It is an acceptable alternative to universal precautions provided that all other portions of the standard are also followed.
Engineering and work practice controls:
The standard requires the employer to use engineering and work practice controls as the primary means of eliminating or minimizing employee exposure. Engineering controls reduce employee exposure in the workplace by either removing or isolating the hazard or isolating the worker from exposure. Work practice controls alter the manner in which a task is performed to make the task safer. When occupational exposure remains after using these controls, the employer must provide, and be sure that employees use, personal protective equipment as additional protection.
Some examples of engineering controls that may be used to reduce exposure to blood or other potentially infectious materials include self-sheathing needles, puncture-resistant containers for the disposal of contaminated sharps, and resuscitation bags and ventilation devices. Examples of work practice controls include prohibiting recapping, removing or bending needles unless no alternative exists; enforcing hand washing procedures following the removal of gloves; restricting eating and drinking in work areas; and decontaminating equipment before servicing.
Handwashing facilities and requirements:
Handwashing facilities must be readily accessible to employees. Handwashing with soap and at least tepid (lukewarm) running water must be performed as soon as feasible to adequately flush contaminated material from the skin. The employer must ensure that handwashing is routinely performed immediately following removal of gloves and other personal protective equipment that have become contaminated. Employers must make handwashing facilities available at a reasonable distance from a work area where exposure may occur. Contamination of surfaces is more likely when employees must travel long distances, through doorways and through stairs to reach handwashing facilities, and is therefore not permitted by the standard.
When handwashing facilities cannot be made available, such as to emergency personnel at accident sites, antiseptic hand cleaners or antiseptic towelettes must be provided. The employee must wash his or her hands or other contaminated skin with running water and soap as soon as possible.
Handling needles and sharps:
Devices exist to provide an alternative to the use of needles for some procedures. Examples of such devices include stopcocks (on-off switches), needle-protected systems or needleless systems to connect intravenous lines, and self-sheathing needles. Needles may be recapped only in very limited situations.
When a procedure requires that the needle be recapped, the employee must use some type of device that protects the hand or allows a safe one-handed recapping method. Finger or hand shields may be used but must be constructed so that the employee is not exposed to a needle protruding from the side or end of the cap. Forceps may also be used, but a onehanded method is required. Shearing or breaking contaminated needles is never permitted.
Personal protective equipment:![]()
When occupational exposure continues after engineering and work practice controls have been instituted, personal protective equipment (PPE) must be used. The PPE must prevent blood or other potentially infectious materials from passing through to or contacting the employees’ work or street clothes, undergarments, skin, eyes, mouth, or other mucous membranes. This barrier must remain intact under normal conditions of use and for the entire time it is being used. The employer must provide appropriate PPE at no cost to the employee. PPE must be cleaned, laundered, disposed of, repaired and replaced at no cost to the employee.
PPE must be provided in appropriate sizes and accessible locations. Training must be given as to what personal protective equipment to use, where it is kept, and how it is properly used.
Housekeeping:
The standard requires employers to ensure that the worksite is maintained in a clean and sanitary condition. An appropriate written schedule for cleaning and a method of decontamination based upon the location within the facility, type of surface to be cleaned, type of soil present, and tasks and procedures must be implemented. The term worksite refers not only to permanent fixed facilities but also covers temporary, nonfixed worksites such as ambulances, bloodmobiles and temporary blood collection centers.
A solution of household bleach (containing 5 percent sodium hypochlorite) diluted with water to a concentration of 1:10 to 1:100 is an effective disinfectant against HBV and HIV. However, it may be corrosive to some equipment and environmental surfaces and therefore may not be an appropriate choice for all situations.
Hepatitis B vaccination:
The standard requires that all employees with reasonably anticipated exposure, regardless of the frequency of exposure, be offered vaccination against the hepatitis B virus. This must take place after the training, and within 10 working days of initial assignment in a covered job description. The vaccine must be provided at no cost to the employee unles.
(1) the employee has previously received the complete hepatitis B vaccination series,
(2) antibody testing reveals the employee is immune, or
(3) medical reasons prevent taking the vaccinations.
An employee may refuse the vaccination, but if he or she does so, the employer must document the refusal by having the employee sign the declination from required by the standard. Employees who decline the vaccine must be allowed the option of having the vaccination at any time so long as they still have occupational exposure.
Post-exposure evaluation and follow-up:
Following the report of any incident in which an employee has non-intact skin, eye, mouth, mucous membrane or parenteral (under the skin) contact with blood or other potentially infectious materials, the employer must provide the employee a confidential medical evaluation and follow-up. This evaluation must be conducted by a licensed health care professional. It must include the following elements:
1. Documentation of the route(s) of exposure and the circumstances under which the exposure occurred.
2. Identification and documentation of the source individual unless the employer documents that such identification is infeasible or prohibited by state or local law.
3. Collection and testing of the exposed employee’s blood for HBV and HIV serological status, which means finding out if the virus is already present in the employee’s blood.
4. Post-exposure prophylaxis, when medically indicated as recommended by the CDC.
5. Counseling: The employer must provide counseling about both the exposure incident and the medical follow-up, and must also provide psychological counseling if it is recommended by the healthcare professional.
6. Evaluation of reported illnesses: The exposed employee should be instructed to report and seek medical evaluation for any acute illness or any illness with a fever that occurs during the follow-up period.
Following the post-exposure follow-up, the health care professional must provide the employer a written opinion including whether the hepatitis B vaccine is indicated and whether the employee received such vaccination. An opinion of what post-exposure evaluation and follow-up is needed. The employer must obtain this report and give a copy to the employee within 15 days after the evaluation is completed.
All other findings or diagnoses must remain confidential and cannot be included in the written report. This provision of confidentiality may become a problem in small medical or dental offices where the employer serves as the health care professional for the employees. Using a physician outside the workplace is recommended in such situations.
Communication of Hazards to Employees:
Labeling requirements:
Warning labels must be placed on containers of regulated waste, refrigerators and freezers containing blood or other potentially infectious materials, and other containers used to store, transport, or ship blood or other potentially infectious materials, with some exceptions as noted below. Labels for contaminated equipment must state which portions of the equipment remain contaminated.
Labels are required to be fluorescent orange or orange-red in color with lettering or symbols in a contrasting color. They are to be attached as close as feasible to the container by string, wire, adhesive or other method that prevents their loss or unintentional removal. Labels must include the following symbol and wording:
Red bags or red containers may be substituted for labels. Labeling is not required for the following:
(1) containers of blood, blood components or blood products that are labeled and have been released for transfusion or other clinical use;
(2) individual containers of blood or other potentially infectious materials that are placed in a labeled larger container during storage, transport, shipment or disposal; and
(3) regulated waste that has been decontaminated.
Training requirements:
Effective training helps to ensure that employees understand the hazards associated with bloodborne pathogens, the modes of transmission, the exposure control plan, the use of engineering controls, work practices and personal protective clothing.
The standard requires that the training be given at the educational level and in the language primarily used by the employees being trained. The training may be specific to different groups being trained. For example, doctors and nurses may require less information on the causes and symptoms of bloodborne pathogens than would laundry workers. All training must take place during working hours, at no cost to the employee, and in a reasonable, accessible location.
Training must be provided at the time of initial employment and at least annually (once a year) after that. When a worker’s job is changed to include different ways to do tasks or procedures, or when new tasks or procedures are added that affect the employee’s occupational exposure, additional training is required. The standard specifies that the person conducting the training must be knowledgeable in the subject matter in the standard as it relates to the workplace where the employee(s) will be working. The employer should document (keep a written record of) training that the trainer has received in the area of bloodborne pathogens, as well as familiarity with the workplace involved.
The following elements must be included in the training program, at a minimum:
1. A copy of the standard must be made available where each employee has access to it. The contents must be explained as part of the training.
2. A general explanation of the causes and symptoms of bloodborne diseases must be given.
3. The program must cover how bloodborne pathogens are communicated from one person to another.
4. The exposure control plan must be explained in a way that each employee can understand it. The plan must be kept available where each employee has access to it.
5. Employees must be given information on the appropriate methods for recognizing tasks and other activities that may involve exposure to blood and other potentially infectious materials.
6. The use and limitations of methods that will prevent or reduce exposure must be covered. This instruction must include appropriate engineering controls, work practices and PPE.
7. The employee must be taught the types, proper use, location, removal, handling, decontamination and disposal of personal protective equipment.
8. An explanation must be given of the basis for selection of PPE, so that the employee will be able to judge the best PPE to use in any situation.
9. The hepatitis B vaccine must be explained in detail, including information of the vaccine’s effectiveness, safety, method of administration, the benefits of being vaccinated and the assurance that the vaccine will be offered free of charge to the employee.
10. Affected employees must be instructed on the appropriate actions to take and people to contact in an emergency involving blood or other potentially infectious materials.
11. Procedures to follow in case of an exposure incident must be outlined, including the method of reporting the incident and the medical follow-up that will be made available.
12. Employees must be given information on the post-exposure evaluation and follow-up that must be provided to them following an exposure incident.
13. An explanation of the signs and labels and the color coding required by the standard must be given.
14. The person conducting the training must provide an opportunity for questions and answers on the training. A video, film or written information by itself is not sufficient for this standard. Similarly, computer-based training is not sufficient by itself unless the employee can have questions and concerns answered by a knowledgeable individual at the time that he or she takes the computer based training.
Blood borne Pathogen Post Exposure Procedures:
• Document the route of exposure and exposure event circumstances
• Identify and document the source individual
• Test the source individual’s blood for HBV and HIV as soon as possible.
• Have your blood tested
• Administer post exposure prophylaxes
• Provide counseling.
• Evaluate reported illnesses.
Occupational exposure can occur through:
• Accidental puncture from contaminated needles, broken glass, or other sharps
• Contact between broken or damaged skin and infected body fluids
• Contact between mucous membranes and infected body fluids Anytime there is blood-to-blood contact with infected blood or body fluids, there is a slight potential for transmission. Unbroken skin forms an impervious barrier against bloodborne pathogens. However, infected blood can enter your system through:
- Open sores

- Cuts
- Abrasions
- Acne
- Any sort of damaged or broken skin such as sunburn or blisters
In an emergency situation involving blood or potentially infectious materials, you should always use Universal Precautions and try to minimize your exposure by wearing gloves, splash goggles, pocket mouth-to-mouth resuscitation masks, and other barrier devices. Exposure – If you are exposed, however, you should: Wash the exposed area thoroughly with soap and running water. Use non-abrasive, antibacterial soap if possible. If blood is splashed in the eye or mucous membrane, flush the affected area with running water for at least 15 minutes.
Training Program Minimum Standards:
Materials must be in a language common to employees and appropriate in content and vocabulary to their educational and literacy levels.
The training program needs to contain, at a minimum, the following elements:
- A general explanation of the epidemiology and symptoms of bloodborne diseases.
- An explanation of the modes of transmission of bloodborne pathogens.
- An explanation of the employer’s exposure control plan and the means by which the employee can obtain a copy of the plan.
- An explanation of how to recognize activities that may involve exposure to blood and other potentially infectious material.
- An explanation of the use and limitations of methods that will prevent or reduce exposure, including appropriate engineering controls, work practices and personal protective equipment (PPE).
- Information on the types, proper use, location, removal, handling, decontamination and disposal of PPE.
- An explanation of the basis for selecting PPE.
- Information on the efficacy, safety, benefits and method of administration of the Hepatitis B vaccine, and its availability, free of charge, to employees with an occupational hazard exposure.
- Information on the appropriate actions to take and people to contact in an emergency involving blood or other potentially infectious material.
- An explanation of the procedure to follow if an exposure incident occurs, including the method of reporting the incident and the medical follow-up that will be made available.
- Information on the post-exposure evaluation and follow-up that the employer is required to provide for the employee following an exposure incident.
- An explanation of the signs and labels and/or color coding required by 29 CFR 1910.1030(g)(1).
- An opportunity for questions and answers with the person conducting the training session.
- An accessible copy of the regulatory text of this standard and an explanation of its contents.
Hazards
Unprotected exposure to body fluids presents the possible risk of infection from a number of bloodborne pathogens notably Hepatitis and HIV.
Hazard Control
Engineering Controls – prevention of exposure to bloodborne pathogens engineering controls include proper storage facilities and containers, syringes designed to prevent accidental needle sticks, autoclaves and disinfectant equipment.
Administrative Controls – prevention of exposure to bloodborne pathogen administrative controls include universal precautions, assignment of PPE, employee training, use of spill kits specifically designed for blood and body fluids, restricted access to waste collection points and waste disposal procedures.
Recordkeeping:
All recordkeeping must be kept in accordance with Title 29 Code of Federal Regulations Part 1910.1020 which governs access to employee exposure and medical records. Upon request, records required by this standard must be made available to the commissioner of labor and to the director of the National Institute for Occupational Safety and Health.
Records must be made available to employees upon request and may also be released to the employees’ representatives. Medical records can be released to people other than an employee only upon that employee’s written consent.
Medical records:
Many diseases, such as silicosis and asbestosis, are found only after many years. Therefore the recordkeeping standard was written to provide medical information about employees even many years after they have left the workplace. It is still not known how long it may be before an infection with the HIV virus becomes AIDS, or whether some people with the virus will ever develop AIDS. Therefore, it seems a wise precaution with this disease to keep such records for a seemingly long period.
The employer must establish and maintain an accurate record for each occupationally exposed employee. These records must be maintained for the period of employment plus 30 years.
The standard requires that the following information be included in the medical record:
1. The name and Social Security number of the employee.
2. A copy of the employee’s hepatitis B vaccination status including the dates of all the hepatitis B vaccinations and any medical records relative to the employee’s ability to receive vaccination.
3. A copy of all results of post-exposure evaluation examinations, medical testing and follow-up procedures.
4. The employer’s copy of the healthcare professional’s written opinion.
5. A copy of the information provided to the healthcare professional.
The employer must ensure that employee medical records are kept confidential and are not disclosed or reported without the employee’s written consent to any person within or outside the workplace except as required by law.
Training records:
Training must be documented in writing. The following information must be included:
1. The dates of the training session.
2. The contents or a summary of the training session.
3. The names and qualifications of people conducting the training.
4. The names and job titles of all people attending the training sessions.
Training records must be maintained for three years from the date on which the training occurred.
Sharps injury log:
Employers who are covered by the Bloodborne Pathogens Standard and who are required by Title 29 Part 1904 to maintain occupational injury and illness records must maintain a sharps injury log for recording all percutaneous (skin piercing) injuries from contaminated sharps. The information in the sharps injury log must be recorded and maintained in such a manner as to protect the confidentiality of the injured employee.
The sharps injury log, at a minimum, must contain:
1. The type and brand of device involved in the incident,
2. The department or work area where the exposure occurred, and
3. An explanation of how the incident occurred.
The sharps injury log must be maintained for five years following the year to which it applies.
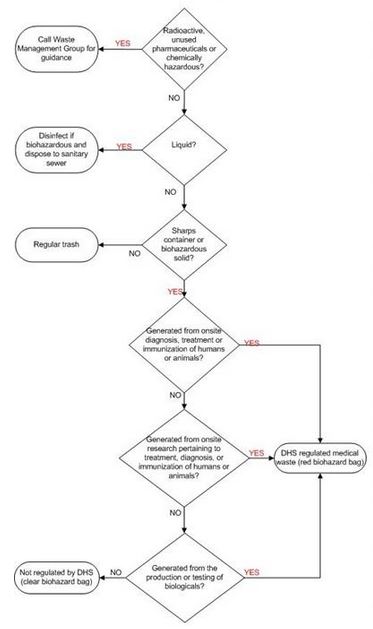
Medical Wastes:
Medical/infectious waste must be segregated from other waste at the point of origin.
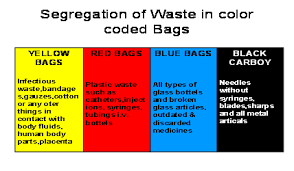
Medical/infectious waste, except for sharps (i.e., razor blades, broken glass, needles, etc.) capable of puncturing or cutting, must be contained in double disposable red bags conspicuously labeled with the words “INFECTIOUS WASTE” and “BIOHAZARD.”
 Used needles or other sharps (razor blades, broken glass, scalpels, etc.) must not be sheared, bent, broken, recapped, or resheathed. Infectious sharps must be contained for disposal in leak-proof, rigid puncture-resistant containers. Infectious waste contained as described above must be placed in reusable or disposable leak-proof bins or barrels that are conspicuously labeled with the words “INFECTIOUS WASTE” and “BIOHAZARD.” These waste barrels are picked up regularly by an outside company licensed to handle infectious wastes.
Used needles or other sharps (razor blades, broken glass, scalpels, etc.) must not be sheared, bent, broken, recapped, or resheathed. Infectious sharps must be contained for disposal in leak-proof, rigid puncture-resistant containers. Infectious waste contained as described above must be placed in reusable or disposable leak-proof bins or barrels that are conspicuously labeled with the words “INFECTIOUS WASTE” and “BIOHAZARD.” These waste barrels are picked up regularly by an outside company licensed to handle infectious wastes.
All infectious agents, equipment, or apparatus must be disinfected in an autoclave or otherwise disinfected before being washed or disposed of. Each individual working with infectious bio-hazardous agents is responsible for dis-infection and disposal of these agents.
Biological wastes that do not contain radioactive or hazardous substances may be disinfected by steam sterilization (autoclave) then disposed of in the regular trash.
Liquid bio-hazardous waste may be disposed of in the sewage system following chemical decontamination. Reusable glassware must be decontaminated in sodium hypo chlorite (household bleach) solution (1:9) prior to rinsing and acid washing. The glassware must then be sterilized in an autoclave.
To minimize the hazard to firefighters or emergency response personnel, at the close of each work day and before the building is closed, all infectious or toxic material must be placed in a refrigerator, placed in an incubator, or autoclaved or otherwise disinfected.
Infectious agents must not be placed in an autoclave and left overnight in anticipation of auto claving the next day. Floors, laboratory benches, and other surfaces in buildings where infectious agents are handled must be disinfected with a suitable germicide, such as 1:9 sodium hypo chloride solution (household bleach) as often as necessary as determined by the supervisor.
The surroundings must be disinfected after completion of operations involving planting, pipetting, centrifuging, and similar procedures with infectious agents. Infectious agents must not be dumped into the building drainage system without prior disinfection.
Cuts
If an employee has a needle stick, cut, or mucous membrane exposure to another persons body fluids he/she must report the incident immediately to the Company Nurse. Blood Exposure All employees exposed to human blood and blood products must report to the Company Nurse for information and possible inclusion in the Hepatitis B Immunization Program.
Infection Control Plan
The purpose of the Infection Control Plan is to protect the health and safety of the persons directly involved in handling the materials, Company personnel and the general public by ensuring the safe handling, storage, use, processing, and disposal of infectious medical waste. This plan complies with OSHA requirement proposed for 29 CFR 1910.1030, Blood borne Pathogens.
Universal precautions: Refers to a system of infectious disease control which assumes that every direct contact with body fluids is infectious and requires every employee exposed to be protected as though such body fluids were infected with blood-borne pathogens. All infectious/medical material must be handled according to Universal Precautions (OSHA Instruction CPL 2-2.44A).
The following universal precautions must be taken.
1. Gloves must be made of appropriate disposable material, usually intact latex or vinyl. They must be used:
- when the employee has cuts, abraded skin, chapped hands, dermatitis, or the like.
- when examining abraded or non-intact skin of a patient with active bleeding. c. while handling blood or blood products or other body secretions during routine procedures.
2. Gowns, aprons, or lab coats must be worn when splashes of body fluid on skin or clothing are possible.
3. Mask and eye protection are required when contact of mucosal membranes (eyes, mouth or nose) with body.
Click here to download the blood borne control plan audit check sheet
blood-borne-contrl-plan-check-sheet